It has been approved with the National Class II Medical Device Production License, No.: Qiong Yao Jian Xie Sheng Chan Xu No. 20230001
Zhengyu Biotechnology (Hainan) Co., Ltd. is a wholly-owned subsidiary of Shandong Hengyu Biopharmaceutical Co., Ltd. Hengyu Group, the headquarter, invested 80 million yuan in Haikou City, Hainan Province for the first batch, shouldering the important task of Hengyu Biological's expanding domestic and international production demand and the company's international strategic layout.
Click the lower right corner of the video to view it in full screen
Source factory of high-end medical devices
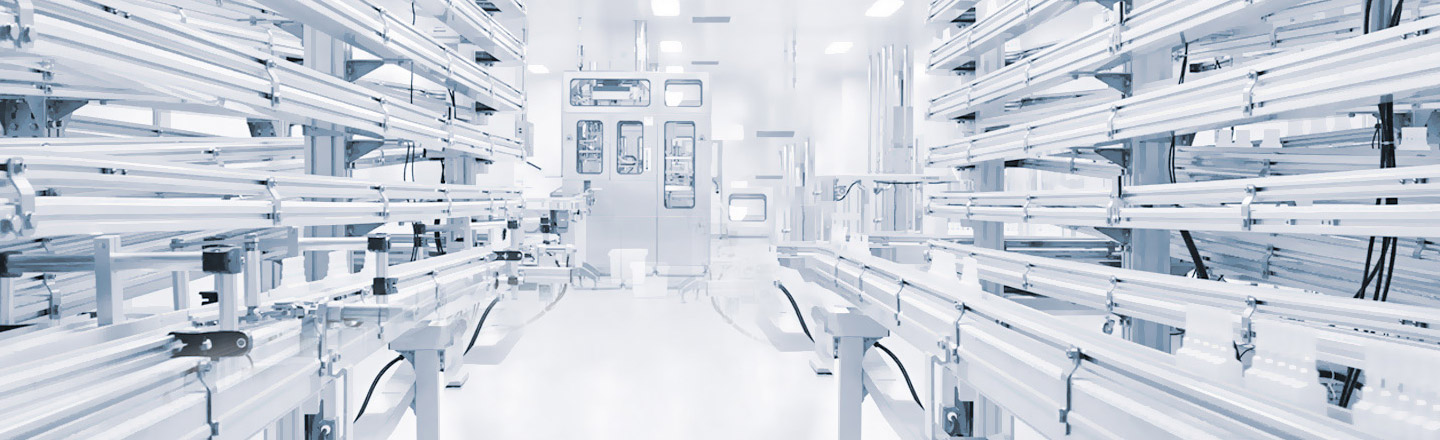
Zhengyu Biotechnology is a high-tech enterprise integrating product development, registration, production, and application. Supporting standardized production workshop; GMP standard modern production line operation; 1 billion/year production capacity, including R&D and production of dosage forms such as application, spray, gel, milk and pre-potting. The annual production capacity exceeds 40 million pieces, and the B+A level is upgraded, which can fully guarantee the production requirements and production capacity of Class I, II and III medical devices.
At the same time, there is a research and development experimental platform, covering product formula research and experimental functions such as preparation and purification research room, drug formulation research room, drug analysis research room, pharmacological research room, etc.
PDRN/PN International Trade Pioneer
Zhengyu Biotechnology, with the help of scientific research endorsement and production line support and in combination with the trade "bridgehead" advantages of Hainan Free Trade Port, the policy advantages of "National Nine Rules" of Hainan Special Administrative Region and Boao Medical Pioneer Zone and a series of tax supports for medicine and medical devices, focuses on the import and export of PDRN/PN international high-end medical equipment, production, qualification approval of product and the landing of international innovative products, and at present, Zhengyu Biotechnology is taking the lead in the registration and filing of PDRN/PN Class III device.



Link and view:
● June 1, 2020
The Overall Plan for the Construction of Hainan Free Trade Port was released.
●December 31, 2020
With the approval of the State Council, the National Development and Reform Commission and the Ministry of Commerce issued the full text of the Measures for the Special Administrative of Foreign Investment Access in Hainan Free Trade Port (Negative List) (2020 Version), which shall come into force on February 1, 2021.
●April 8, 2021
The National Development and Reform Commission, the Ministry of Commerce and other ministries and commissions jointly issued the Opinions on Several Special Measures to Support the Construction of Hainan Free Trade Port to Relax Market Access, including 22 specific measures in five major fields, including medical, financial and cultural.
●May 6, 2021
The First China International Consumer Goods Expo opened in Haikou.
●June 10, 2021
The 29th Meeting of the Standing Committee of the 13th National People's Congress passed the Laws of Hainan Free Trade Port of the People's Republic of China through voting
●August 26, 2021
The Special Administrative Measures for Cross border Service Trade in Hainan Free Trade Port (Negative List) (2021 Version) issued by the Ministry of Commerce was officially implemented. It is the first negative list of cross-border service trade in China.
●February 10, 2022
The head of the National Development and Reform Commission presided over the opening meeting of the preparatory work for the island wide closure of Hainan Free Trade Port.
●April 10-13, 2022
Xi Jinping Visits Hainan: Accelerating the Construction of a Free Trade Port with Chinese Characteristics with World Influence.
●March 28-31, 2023
Boao Forum for Asia